
The division conducts promising state-of-the-art research to generate new knowledge and improve understanding with the ultimate goal of applying the latest scientific and medical advances for the prevention, detection, and treatment of patients with endocrine diseases.
The focus of Dr. Alonso’s lab is to understand the molecular pathways that trigger pancreatic beta cell proliferation, to find approaches to make more beta cells to prevent or treat diabetes. Using knockout and transgenic mice, and human and mouse pancreatic tissue in culture systems, she is particularly interested in the intriguing relationship between the beta cell workload response and generation of new beta cells.
Clinical & Translational Science Center
Endocrinologist Dr. Julianne Imperato-McGinley leads the Clinical & Translation Science Center (CTSC), an NIH-funded $49 million Clinical & Translation Science Award (CTSA).

The Comprehensive Weight Control Center, led by Dr Louis Aronne and Dr Alpana Shukla, is focused on innovative clinical research in obesity and type 2 diabetes. The center has performed over 50 clinical trials (Phase II and Phase III) of pharmacologic agents and medical weight loss devices. The center's investigator-initiated research has provided novel insight on the impact of food order/nutrient sequencing during a meal on postprandial glucose levels. Ongoing studies are investigating the practical feasibility and effectiveness of this intervention in individuals with prediabetes and diabetes. The center provides training and research mentorship for graduate and medical students, as well as residents and fellows with an interest in obesity/metabolic research.
The Reilly lab is focused on understanding the pathways that regulate energy storage versus energy expenditure in adipocytes. Knockout and transgenic mice are utilized to study physiology and cellular signaling systemically and in isolated primary cells, connecting adipocyte signaling and metabolism to whole body energy balance, obesity and obesity associated diseases.

Dr. Sharma’s lab focuses on novel pathways regulating pancreatic beta cell mass and function. Beta cell stress, specifically ER stress, has been implicated in loss of beta cell mass and function in both T1D and T2D. Increased metabolic load can lead to increased beta cell mass in mice and humans, but failure to adapt to this metabolic insult results in diabetes. Utilizing transgenic mouse models and human islets, he seeks to understand the mechanisms regulating this delicate balance and the tipping point from adaptation to failure. If this process is dynamic and reversible, better understanding it could lead to novel therapies aiming directly at beta cell ER stress to overcome diabetes.
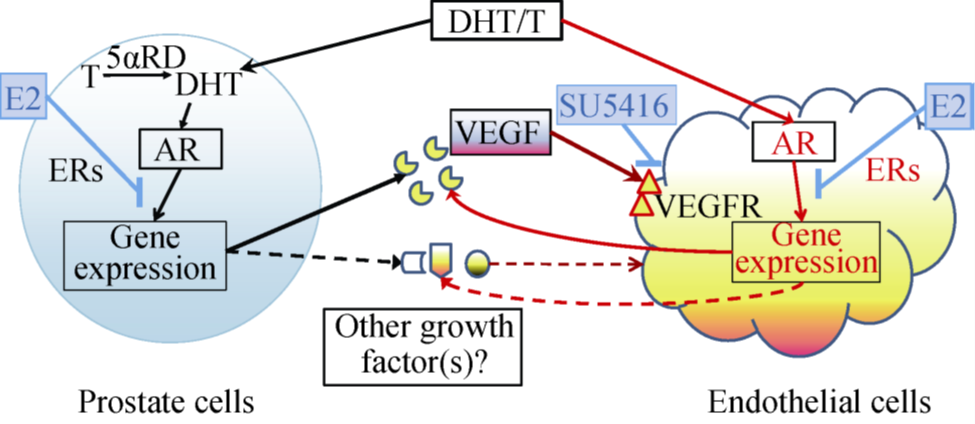
The Zhu lab uses both in vitro cell cultures and in vivo animal models to elucidate the effects and molecular mechanisms of estrogen receptor ligands on modulation of androgen actions and on prostate cancer therapy. Dr. Zhu also directs the CTSC core laboratory to develop/validate biomarker testing and provide services to investigators in sample processing, storage, testing in biochemistry, cell biology, cytokines, endocrine, molecular biology and genetics, and 3D printing and scanning. (Pictured: An illustration of estrogen (E2) modulation of androgen (T/DHT) actions in the prostate and endothelial cells.)