Dr. David Artis and colleagues have published breakthrough findings in Nature that have opened a newly targeted pathway, and ultimately, a new approach for the treatment of obesity and obesity-related diseases. Utilizing a wide array of high tech tools, the team discovered for the first time that ILC2s, a group of innate lymphoid cells, promote the "beiging" of white adipose tissue (a type of body fat) and limit obesity.
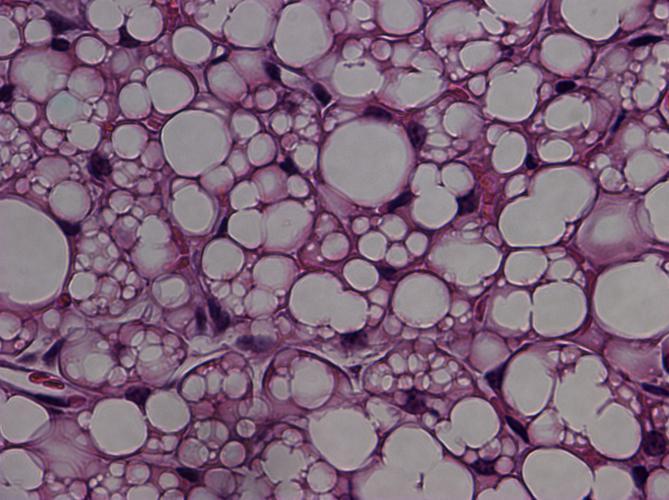
Histologic image showing characteristic murine beige fat cells that are implicated in increasing caloric expenditure and limiting weight gain. (Image courtesy of Jonathan R. Brestoff and David Artis.)
In recent years, obesity has been deemed a disease category in the medical literature. It is considered to be at the root of many debilitating medical conditions, and the NIH's National Health and Nutrition Examination Surveys (NHANES, 2009-2010) estimate that approximately 69% of American adults are overweight or obese. Dr. Artis, a world-renowned researcher in the field of immunology, and the Director of the Jill Roberts Institute for Research in Inflammatory Bowel Disease (IBD) at Weill Cornell Medicine, states, "In addition to environmental and genetic factors, changes in the immune system have been associated with the development of obesity. In our new paper we have identified that fat tissues of humans and mice are populated by a cell type called innate lymphoid cells, specifically ILC2s, and that these cells become dysregulated when obesity develops. Our study indicates that this type of cell can prevent the development of obesity through the production of bioactive peptides."
The paper that Dr. Artis and colleagues have published in Nature, entitled "Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity," focuses on the "beiging" of white adipose tissue (body fat) as it relates to immunological processes involving ILC2s and the protein IL-33. "Beiging" is a term that refers to an immune process involving the beiging of white fat tissue. When white fat tissue becomes beige or brown, it appears to become resistant to developing obesity. It had been known previously that the emerging cell type, beige adipocyte, produces heat in a thermogenic process that expends calories and is dependent upon the uncoupling of a certain protein.
Using a murine model, the Artis lab initially found that IL-33 could be critical in regulating beiging, and, in particular, can promote beiging in white adipose tissue. Employing genome-wide transcriptional profiling and flow cytometric analysis, the team proceeded to prove that beiging induced by IL-33 is an interaction critically dependent upon ILC2s. In a comparison study, they identified one gene, PCSK1, to be significantly enriched in ILC2s, and that loss of function involving PCSK1 is associated with increased susceptibility to obesity and decreased caloric expenditure. The good news, however, is that the team went on to uncover that ILC2s produce bioactive peptides (enkephalin) that serve to prevent the proclivity to developing obesity.
For the first time, the Artis laboratory has described how the enkephalin peptide effector mechanism is employed by ILC2s to regulate metabolic homeostasis, thus playing a vital role in preventing obesity. Understanding of this innate immune function of the ILC2 cells is now driving a new line of study for targeting the IL-33/ILC2/beiging pathway. Future discoveries will ultimately yield new approaches for the treatment of obesity and its associated diseases, including heart disease, high blood pressure, stroke, type 2 diabetes, metabolic syndrome, and more.
Dr. Artis added, "Of course, we are just at the beginning of these studies and there is a lot more work to be done. However, the identification that the immune system controls beiging of white fat could offer new therapeutic approaches to treat and prevent obesity and obesity-associated diseases."
Research in the Artis lab is supported by the National Institutes of Health, the Burroughs Wellcome Fund and the Crohn's and Colitis Foundation of America. Dr. Artis is a Professor of Immunology in the Division of Gastroenterology and Hepatology and he holds the Michael Kors Professorship in Immunology at Weill Cornell Medicine.

