
Dr. Matthew Simon
Matthew S. Simon, M.D., M.S., Assistant Professor of Medicine (Division of Hospital Medicine), and colleagues, have published a research study on the cost-effectiveness of novel screening strategies for preventing transfusion-transmitted babesiosis, the most common red blood cell transfusion-transmitted infection in the United States. The results suggest that, in highly endemic states such as New York, screening donated blood for antibodies to Babesia, would avoid additional cases and would be cost-effective relative to currently implemented blood supply screening for infections, such as West Nile virus. The authors used a computer simulation model to analyze how laboratory screening tests would affect societal costs and the quality of life of transfusion recipients and blood donors. The new tests were compared to the current screening method, which is to ask potential blood donors if they have a history of babesiosis on a pre-donation questionnaire and which is largely ineffective in identifying infected donors. The study, published in the journal Transfusion was conducted in collaboration with investigators in the WCMC Division of Infectious Diseases, the WCMC Department of Healthcare Policy and Research, the WCMC Department of Pathology and the New York Blood Center.
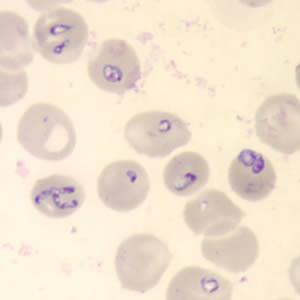
Babesia
Babesiosis is a parasitic infection of red blood cells that is commonly transmitted through a tick bite. Healthy adults can be silent carriers and transmit the parasite after donating blood to immune-compromised transfusion recipients who are often at high risk for severe complication and death from babesiosis. In highly prevalent regions in the Northeast and Upper Midwest, up to two percent of blood donations can contain antibodies to Babesia, and infections may occur anywhere from one in 20,000 and one in 100,000 transfusions. Currently there is no Food and Drug Administration licensed blood supply screening test for Babesia, but several assays are under investigation. The study aims to assist policy makers in addressing the appropriateness of laboratory-based screening of the blood supply for Babesia in various geographic regions of the United States and support implementation of antibody testing in highly endemic areas.

